Atoms are the fundamental units of matter, making up everything in the universe. At one point, atoms were thought to be the smallest indivisible components of all substances. However, modern science reveals that atoms are made up of even smaller particles: protons, neutrons, and electrons. These particles are governed by the laws of physics and chemistry, whether in living organisms or nonliving matter.
Key Takeaways:
- Atoms are the smallest units of matter and retain all the chemical properties of an element.
- Elements are made up of only one type of atom and are the building blocks of matter.
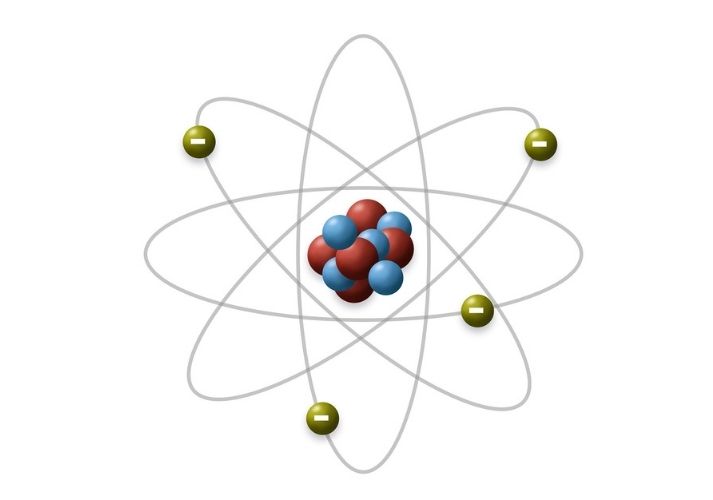
- The Atom’s Structure: An atom consists of a tiny nucleus (containing protons and neutrons) and a cloud of electrons that orbit around the nucleus.
- Subatomic Particles:
- Protons are positively charged and determine the element's atomic number.
- Neutrons are neutral and have almost the same mass as protons.
- Electrons are negatively charged and orbit the nucleus in specific shells.
The Role of Atoms in the Universe
The universe, formed after the Big Bang, began with the creation of atoms. These atoms eventually combined to form stars, planets, and, through the processes of nuclear fusion and decay, more complex elements and compounds. Atoms make up all matter, and their interactions dictate the behavior of substances, whether they are part of a living organism or not.
The Periodic Table and Chemical Behavior
Each element is identified by its atomic number, the number of protons in its nucleus. The periodic table organizes elements based on their atomic number, and this arrangement helps predict their chemical properties. Atoms with similar electron configurations in their outer shells behave in similar ways chemically.
Atoms and Life
The human body is composed of trillions of atoms, with oxygen, carbon, hydrogen, and nitrogen making up most of our composition. These atoms combine into molecules, which form the cells, tissues, and organs that make life possible. The processes that occur within these molecules, governed by the interactions between atoms, are essential for life.
The History of Atomic Theory
The concept of the atom has evolved over centuries. The Greek philosopher Democritus first proposed that matter is composed of indivisible particles, which he called "atoms." This idea was further developed by scientists like John Dalton, who in the early 19th century proposed that atoms of different elements combine in fixed ratios to form compounds.
Conclusion: The Foundation of Everything
Atoms are at the core of everything in the universe. From the air we breathe to the stars in the sky, atoms are the basic building blocks of matter. Understanding atoms and their behavior is essential to comprehending chemistry, physics, biology, and the very nature of reality itself.